





|
Saflo™ Needle Safe Subcutaneous Infusion Set
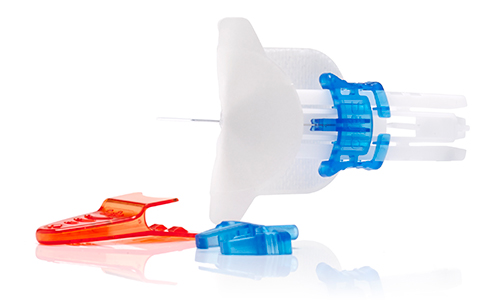 A new design created specifically to meet the requirements of the EU directive on prevention of sharps injury brought into force in May 2013.
The system incorporates a unique selection of connector tubing configurations to meet the widest range of clinical needs. Features
Product Matrix
The table below shows the product options available for the Saflo™ product range. All the connector options are based upon the common primary connection provided by the standard catheter assembly. This will make it possible to choose the best option to meet the changing clinical and social needs of your patients across the duration of their treatments.
|

